Get personalized genetic insights and tools that can help make it easier for you to take action on your health.
- 150+ personalized reports
- Includes Ancestry Service
- Includes FDA-authorized reports
- FSA/HSA eligibility♦Learn about Important Information for FSA/HSA Reimbursement
There is a lot to consider with genetic testing. We encourage you to review relevant information about Carrier Status*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks and Genetic Health Risk*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks reports.
Important test infoDiscover how your DNA can influence your health.
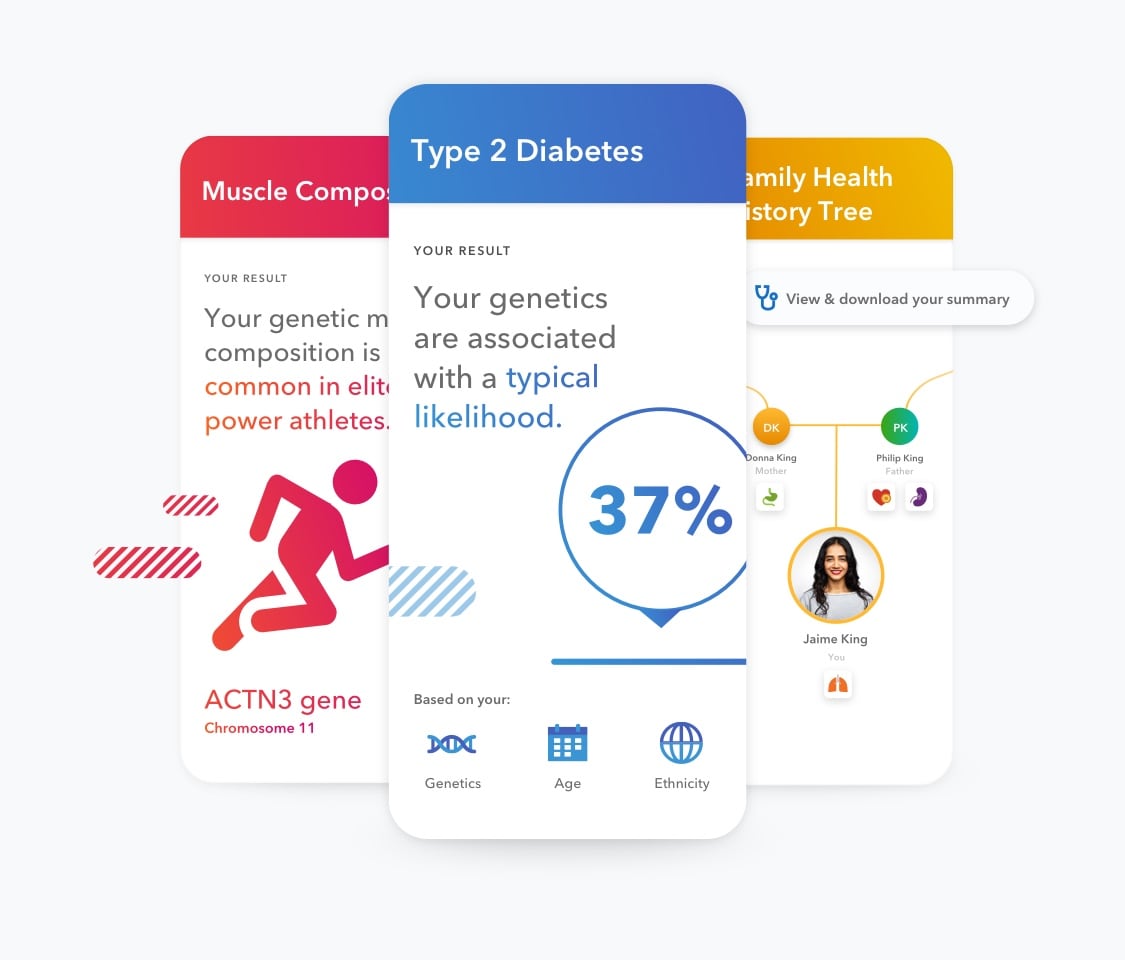
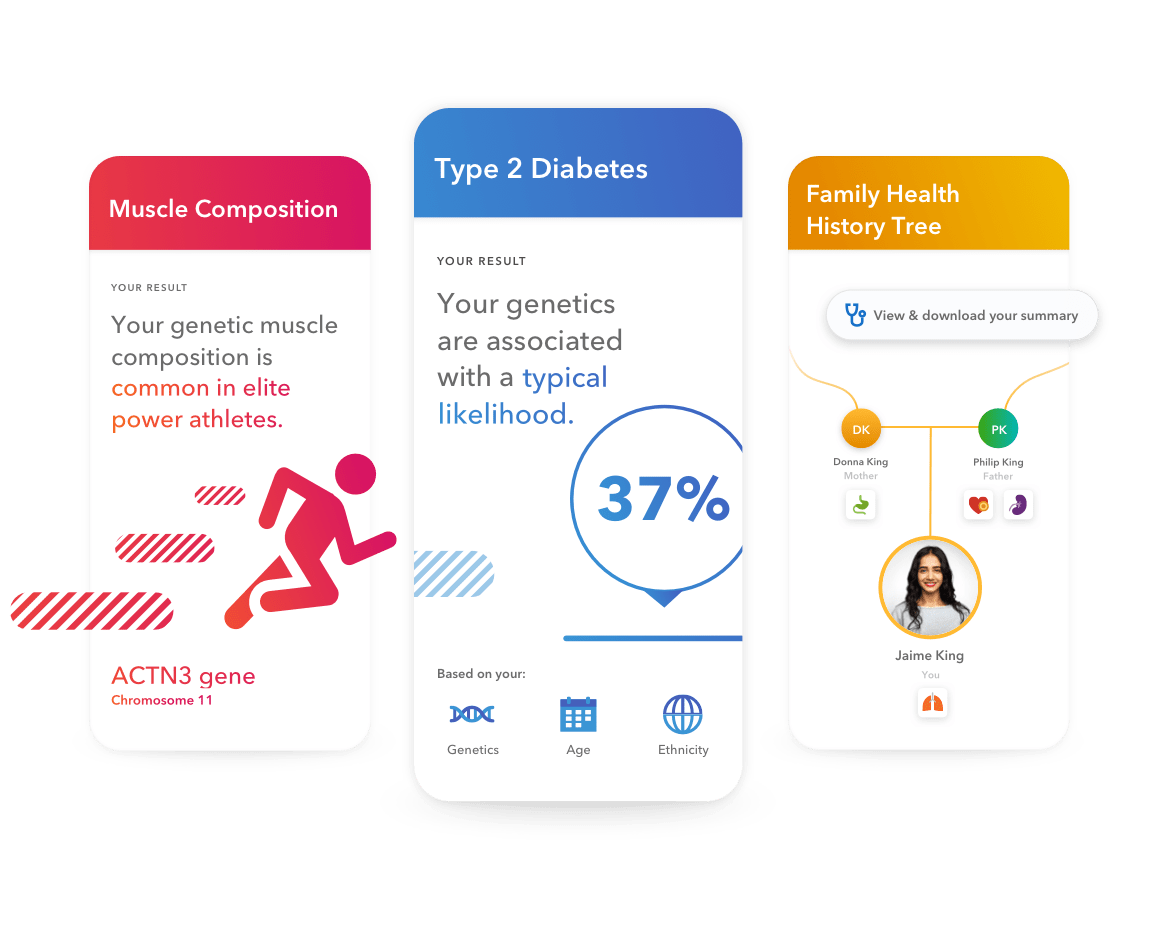
Using insights backed by the latest science, see how your DNA can affect your chances of developing certain health conditions. Your personalized reports break down your genetic data, the science and potential next steps.
(Powered by 23andMe
Research)Reports and features that are “Powered by 23andMe Research” are developed by 23andMe scientists using data and insights gathered from thousands of customers who have consented to participate in our research.(Selected Variants)
Type 2 Diabetes (Powered by 23andMe
Research)Reports and features that are “Powered by 23andMe Research” are developed by 23andMe scientists using data and insights gathered from thousands of customers who have consented to participate in our research.
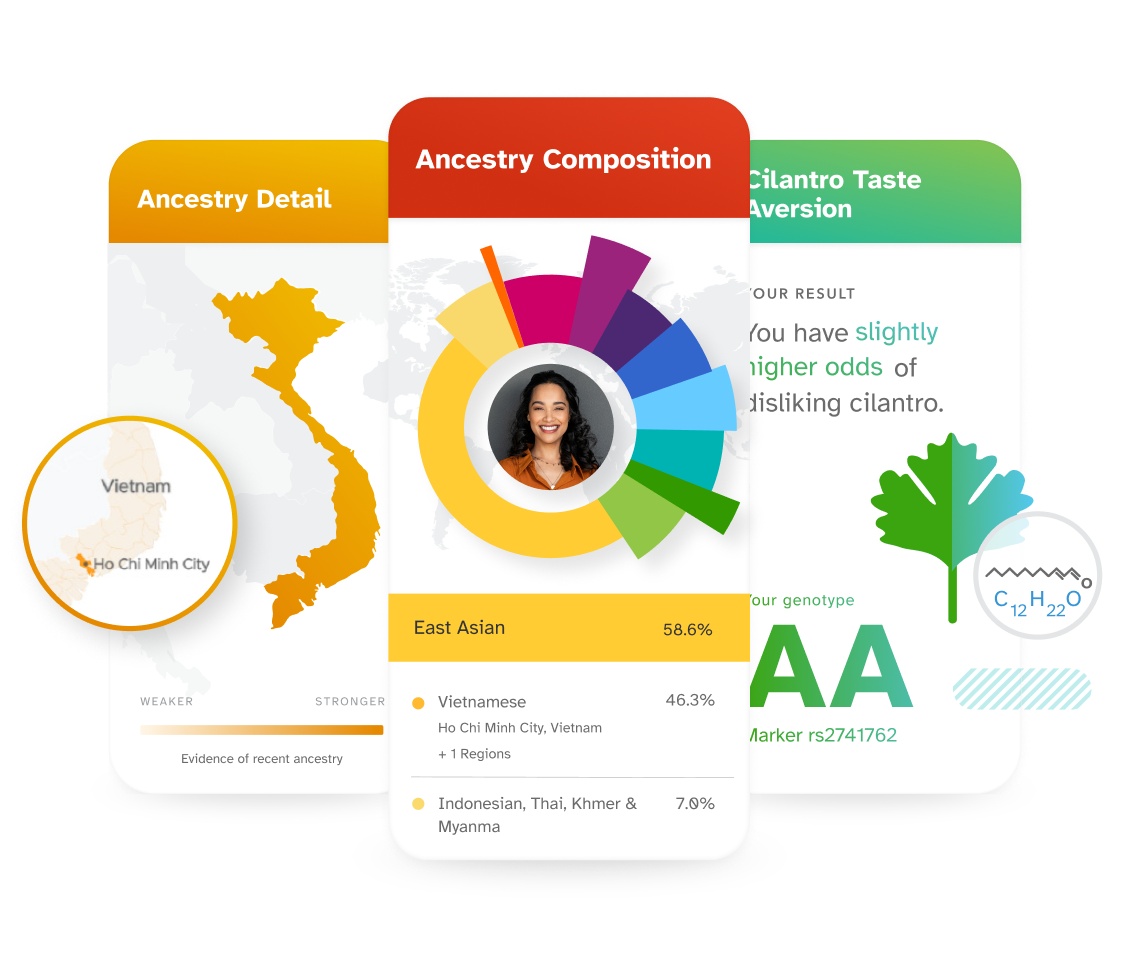
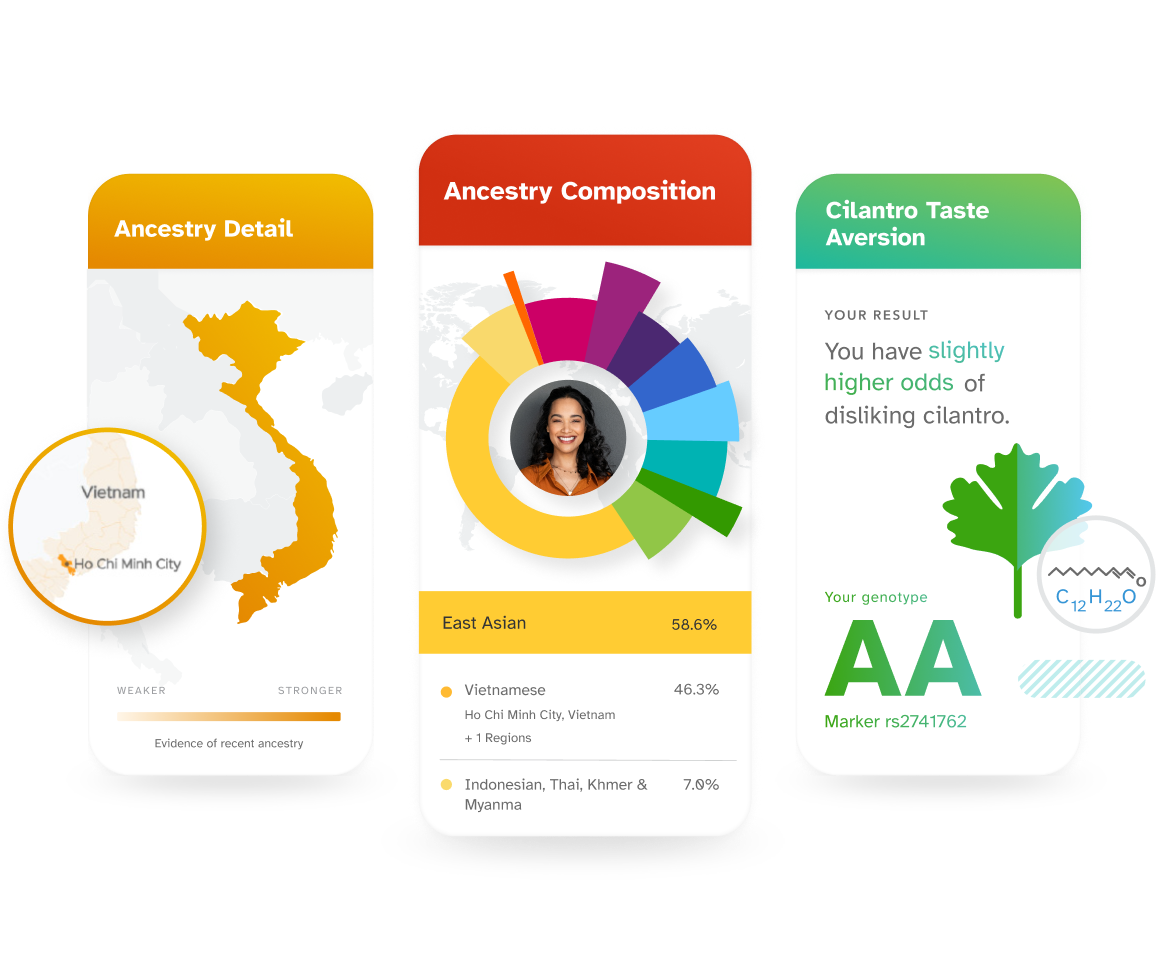
Explore how your DNA relates to your lifestyle.
Discover what your DNA has to say about lifestyle factors like diet, exercise, and sleep. Use what you've learned to help you make informed decisions.
Muscle Composition
See what you may pass on to your future children.
If you're thinking of starting a family, find out if you're a carrier for genetic variants linked to certain inherited health conditions.
Cystic Fibrosis
Which service will you start with today?
Remember, you can always upgrade without having to spit again.
| Feature | Ancestry Service | Health plus Ancestry Service |
|---|---|---|
| Genotyping reports Genotyping examines DNA variants at certain pre-identified positions in the genome. The specific variants we look at generally provide coverage of commonly known variations across the entire genome. | 80+ | 150+ |
Just Added Exome Sequencing Reports Exome sequencing is an advanced genetic testing technique that focuses on decoding the exome of an individual's genome. The exome represents the protein-coding regions of genes, which make up only about 2% of the entire genome but contain the majority of genetic variants associated with disease risk. | ||
| Ancestry and Trait Reports | ||
| DNA Relative Finder and Family Tree (Opt in) | ||
| Health Predisposition reports*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks Learn how your genetics can influence your chances of developing certain health conditions. | 10+ | |
| Carrier Status reports*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks If you are starting a family, find out if you are a carrier for certain inherited conditions. | ||
| Wellness reports Learn how your genes play a role in your well-being and lifestyle choices. | 5+ | |
| Family Health History Tree Opt in to easily input, track and download your family health history to share with your healthcare provider. | ||
| Pharmacogenetics reports**Learn about Considerations and Limitations for Pharmacogenetics Reports Discover how your DNA may impact how your body processes certain medications with three new Pharmacogenetics reports. | ||
| Enhanced ancestry features Get advanced filtering for DNA Relative Finder and access up to 5000 DNA relatives. | ||
Just Added Historical Matches Uncover your historical and ancient relatives, linking you to the past. | ||
| Ongoing new reports and features Get access to new premium reports and features throughout the year. | ||
Just Added Health TracksSM See how making healthy choices each day can greatly impact your health over time | ||
Just Added Health Action Plan Personalized and ongoing recommendations based on genetic and non-genetic data. | ||
Just Added Blood Testing Eligible participants may order in-person blood testing initiated by a clinician and get results in the 23andMe app. | ||
Just Added Genetics-informed clinical care Access to clinicians with training in genetics. Includes unlimited direct messaging, plus a dedicated virtual consultation annually. |
Putting insights into action
As more and more people choose DNA testing for health reasons, they're sharing more and more inspiring stories. These are just a few of the stories of 23andMe Health + Ancestry customers who used what they learned from their DNA to take a more proactive approach to their health.

Watch Charlie’s story
Uncovering a truth

Watch Kristin’s story
Taking action
Don’t take our word for it...
Frequently Asked Questions
23andMe offers a variety of health reports like Type 2 Diabetes (Powered by 23andMe Research), Celiac Disease Genetic Health Risk Report*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks, BRCA1/BRCA2 (Selected Variants) Genetic Health Risk Report*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks and many more. to see a list of all the reports offered.
Your 23andMe Health reports can tell you how your DNA can impact your chances of developing certain conditions. Wellness reports can help you discover what your DNA has to say about lifestyle factors like diet, exercise, and sleep. And if you're thinking of starting a family, our Carrier Status reports can tell you if you're a carrier for genetic variants linked to certain inherited health conditions.*Learn about Considerations and Limitations for Health Predispositions Reports, Carrier Status Reports and Genetic Health Risks
No, 23andMe reports do not diagnose any health conditions or provide medical advice. While having a particular genetic variant can be linked to a higher risk for a condition, it does not necessarily mean you will develop the condition. It is also important to remember that these reports do not cover all possible genetic variants that could influence risk. Other non-genetic factors, such as environment and lifestyle, can also influence risk for these conditions. We recommend that you consult with a healthcare professional if a condition runs in your family, you think you might have the condition or you have questions about any genetic or non-genetic risk factors you may have.
Stay in the know.
Keep up-to-date with new discoveries and exclusive promotions on our DNA testing kits and services.
*The 23andMe PGS test includes health predisposition and carrier status reports. Health predisposition reports include both reports that meet FDA requirements for genetic health risks and reports which are based on 23andMe research and have not been reviewed by the FDA. The test uses qualitative genotyping to detect select clinically relevant variants in the genomic DNA of adults from saliva for the purpose of reporting and interpreting genetic health risks and reporting carrier status. It is not intended to diagnose any disease. Your ethnicity may affect the relevance of each report and how your genetic health risk results are interpreted. Each genetic health risk report describes if a person has variants associated with a higher risk of developing a disease, but does not describe a person’s overall risk of developing the disease. The test is not intended to tell you anything about your current state of health, or to be used to make medical decisions, including whether or not you should take a medication, how much of a medication you should take, or determine any treatment. Our carrier status reports can be used to determine carrier status, but cannot determine if you have two copies of any genetic variant. These carrier reports are not intended to tell you anything about your risk for developing a disease in the future, the health of your fetus, or your newborn child's risk of developing a particular disease later in life. For certain conditions, we provide a single report that includes information on both carrier status and genetic health risk. Warnings & Limitations: The 23andMe PGS Genetic Health Risk Report for BRCA1/BRCA2 (Selected Variants) is indicated for reporting of 44 variants in the BRCA1 and BRCA2 genes. The report describes if a person's genetic result is associated with an increased risk of developing breast cancer and ovarian cancer and may be associated with an increased risk for prostate cancer, pancreatic cancer, and potentially other cancers. The variants included in this report do not represent the majority of the BRCA1/BRCA2 variants in people of most ethnicities. This report does not include variants in other genes linked to hereditary cancers and the absence of variants included in this report does not rule out the presence of other genetic variants that may impact cancer risk. This report is for over-the-counter use by adults over the age of 18, and provides genetic information to inform discussions with a healthcare professional. The PGS test is not a substitute for visits to a healthcare professional for recommended screenings or appropriate follow-up. Results should be confirmed in a clinical setting before taking any medical action. For important information and limitations regarding each genetic health risk and carrier status report, visit 23andme.com/test-info/
diamondBased on purchase price of $229. Check with your FSA/HSA administrator or your tax professional for confirmation on the specific requirements for individual eligibility and reimbursement, including usage, procedures and qualifications.
**23andMe PGS Pharmacogenetics reports: The 23andMe test uses qualitative genotyping to detect 3 variants in the CYP2C19 gene, 2 variants in the DPYD gene and 1 variant in the SLCO1B1 gene in the genomic DNA of adults from saliva for the purpose of reporting and interpreting information about the processing of certain therapeutics to inform discussions with a healthcare professional. It does not describe if a person will or will not respond to a particular therapeutic. Our CYP2C19 Pharmacogenetics report provides certain information about variants associated with metabolism of some therapeutics and provides interpretive drug information regarding the potential effect of citalopram and clopidogrel therapy. Our SLCO1B1 Pharmacogenetics report provides certain information about variants associated with the processing of some therapeutics and provides interpretive drug information regarding the potential effect of simvastatin therapy. Our DPYD Pharmacogenetics report does not describe the association between detected variants and any specific therapeutic. Results for DPYD and certain CYP2C19 results should be confirmed by an independent genetic test prescribed by your own healthcare provider before taking any medical action. Warning: Test information should not be used to start, stop, or change any course of treatment and does not test for all possible variants that may affect metabolism or protein function. The PGS test is not a substitute for visits to a healthcare professional. Making changes to your current regimen can lead to harmful side effects or reduced intended benefits of your medication, therefore consult with your healthcare professional before taking any medical action. For important information and limitations regarding Pharmacogenetic reports, visit 23andme.com/test-info/pharmacogenetics/