Is Alzheimer’s Genetic?Explore Alzheimer’s and what your DNA can tell you
Medically reviewed by:
What is Alzheimer’s disease and how common is it?
Alzheimer’s disease is a form of dementia. It’s characterized by progressive memory loss, gradual cognitive decline, and personality changes.
There are two different types of Alzheimer’s disease: late-onset Alzheimer’s disease and early-onset Alzheimer’s disease. Both have symptoms that are much the same.
Some common genetic mutations, like the ε4 variant in the A P O E gene, can lead to a higher susceptibility for late-onset Alzheimer’s. The genetic risk for developing late-onset Alzheimer’s disease is not as strong as it is for the early-onset form, and as the name suggests, symptoms tend to arise at a later age.
Typical signs and symptoms of Alzheimer’s disease:
- Memory loss that worsens over time
- Mood and personality changes
- Trouble planning or solving problems
- Confusion with place or time
- Difficulty performing daily life activities
The big difference between the types of Alzheimer’s is the age at which symptoms begin. Early-onset Alzheimer’s disease begins at a younger age, with many people first showing symptoms in their 30s, 40s, or 50s. The early-onset type is more rare, accounting for 1% or less of Alzheimer’s cases. Late-onset Alzheimer’s disease is the most common form, and symptoms typically begin after age 65.
Late-onset Alzheimer’s disease affects people of all races and ethnicities. One in ten Americans age 65 and older is affected by Alzheimer’s disease. Elderly African Americans and Hispanic/Latin Americans are more likely to develop the condition than people of other races and ethnicities.
A P O E Prevalence by Ethnicity
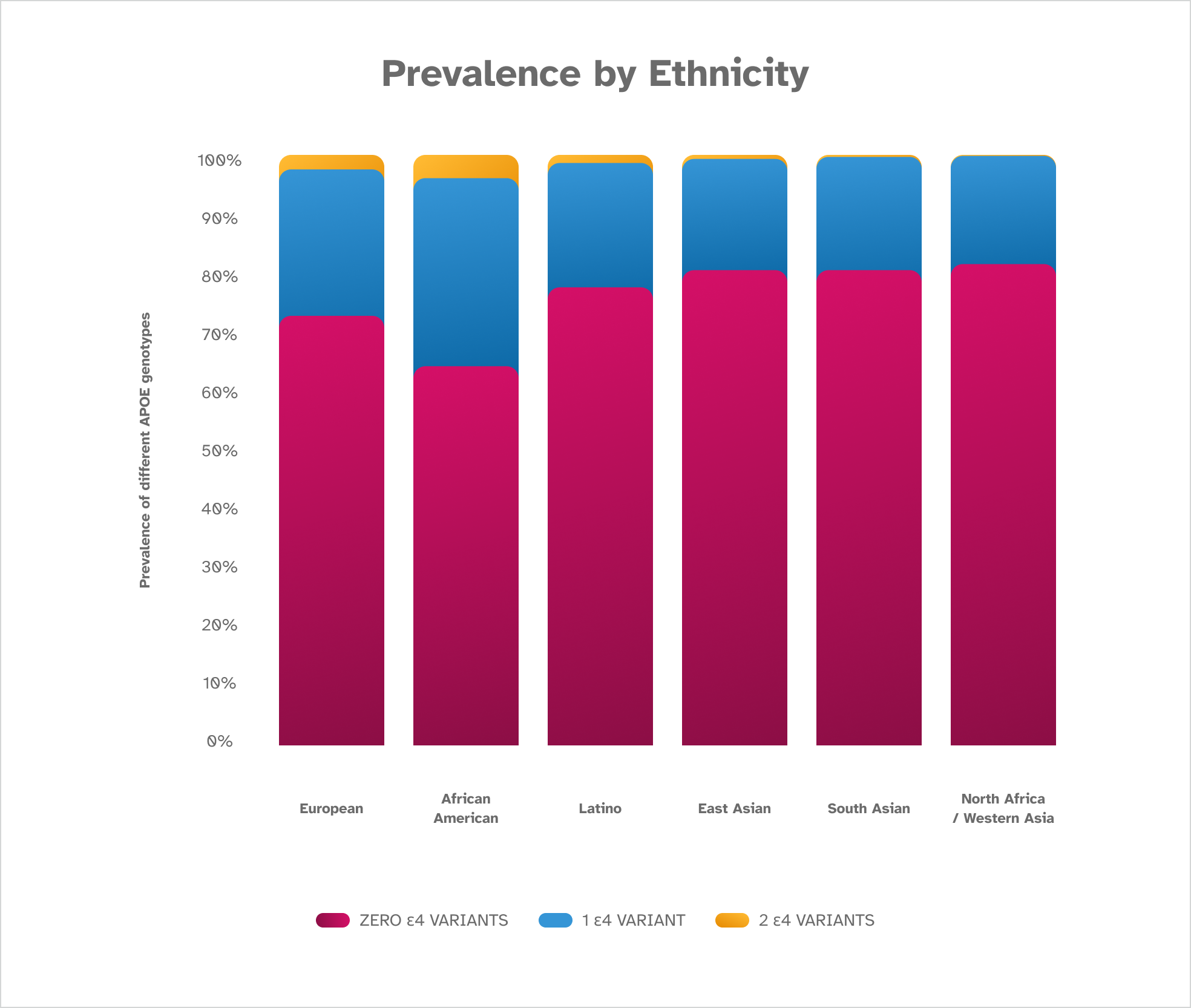
The ε4 variant has been seen in populations worldwide. It is most common in the African American population, followed by those of European descent, and is least common in individuals of South and East Asian descent. The terminology used in this chart to describe populations around the world might differ from what you see on your report. These findings are based on December 2022 data from consented 23andMe research participants.
What causes Alzheimer’s disease?
There is more than one cause of Alzheimer’s disease. In some cases, a faulty gene or group of genetic variants is involved. Families with a strong genetic factor such as that seen in early-onset Alzheimer’s disease show a pattern of inheritance called autosomal dominant inheritance. This means the condition passes directly from parent to child, with each child of an affected parent having a 50% chance of inheriting the gene variant involved in early-onset Alzheimer’s and also being affected by the condition.
Non-genetic factors also play a role in the development of Alzheimer’s. These include age, exercise level, diet, and activities that promote health of the brain and heart. Alzheimer’s disease research focuses on improving the understanding of the interplay between genetic and non-genetic factors, how to reduce risk, and how to slow the progression of Alzheimer’s symptoms.
Recent research from 23andMe shows that adults over the age of 60 with the ε4 variant in the A P O E gene who average at least 30 minutes of exercise per week are less likely to have Alzheimer’s disease, and that holds true for those without the ε4 variant in the A P O E gene too. Talk with a healthcare professional about adding exercise to your routine before you make any major lifestyle changes.
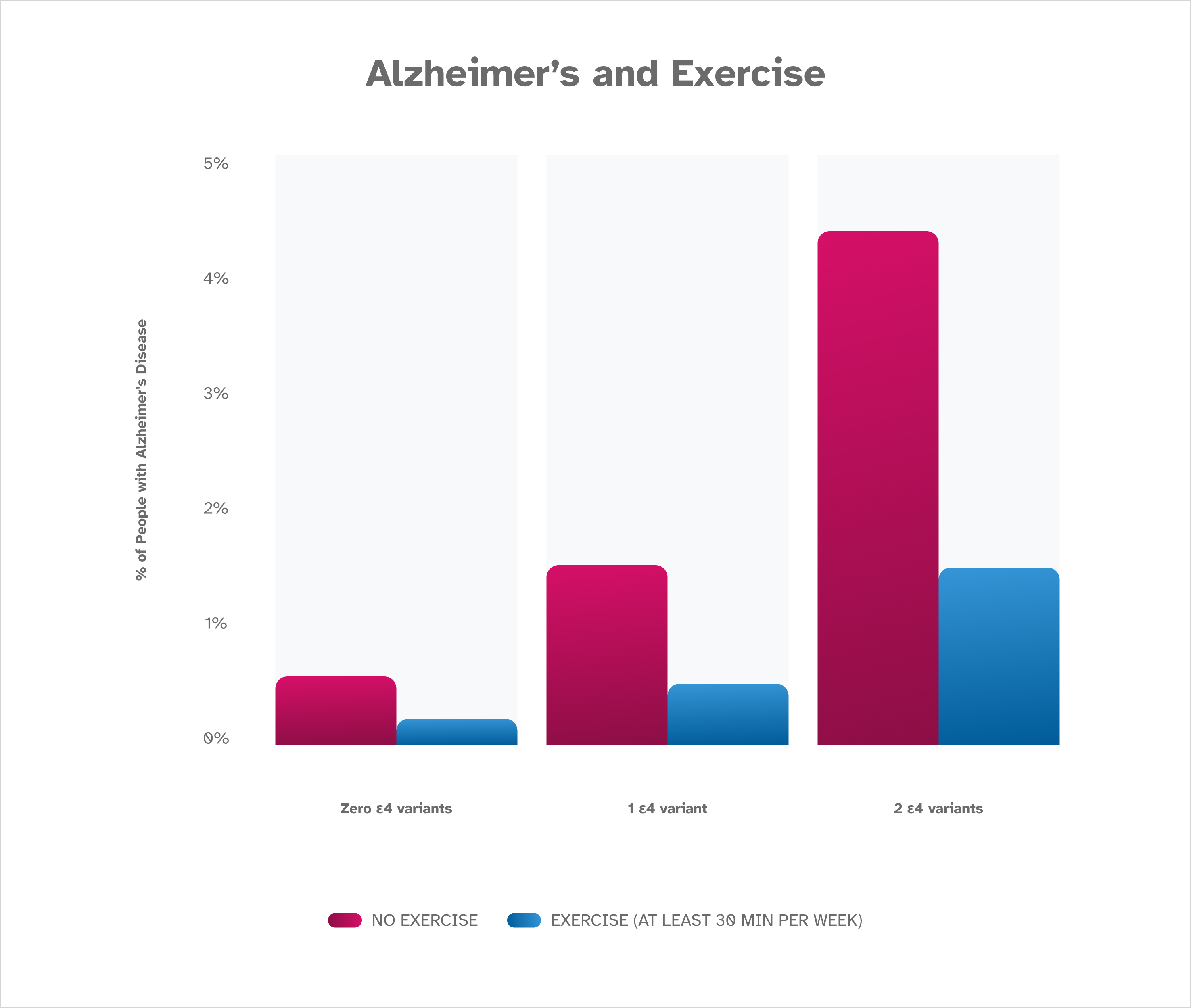
Research by 23andMe shows that people who average at least 30 minutes of exercise per week are less likely to have Alzheimer’s disease. The difference was noted whether participants were found to have zero, one, or two ε4 variants. These findings are based on January 2023 data from about 1,000,000 consented 23andMe research participants over the age of 60.
Is Alzheimer’s disease genetic?
Yes, genetic factors for Alzheimer’s disease have been identified. The ε4 variant in the A P O E gene is the most common genetic variant associated with an increased risk of late-onset Alzheimer’s disease. Having one copy of the ε4 allele increases risk 3-fold, and two copies of ε4 increases risk 8- to 12-fold.
However, ε4 and A P O E are not the only determinants for risk. Some people with Alzheimer’s disease do not have an ε4 allele while some people with one or two copies do not develop disease. There are other genes and genetic mutations that also influence the risk for both late-onset and early-onset forms of Alzheimer’s and non-genetic factors at play.
People with Down syndrome are at a higher risk of developing dementia that is the same as or is similar to Alzheimer’s disease, with 50% of individuals showing signs of dementia by their 60s. This is possibly due to the location of the APP gene on chromosome 21. Researchers are still working to understand the genetic connection between these two conditions.
For all individuals, genetic testing is available to identify variants linked to the different types of Alzheimer’s disease. Additional genetic factors may be discovered in the future.
Does Alzheimer’s disease run in families?
Yes, Alzheimer’s disease can run in families, and this is especially seen in families with genetic variants linked to early-onset Alzheimer’s disease. Also, people with a parent or sibling with Alzheimer’s are more likely to develop the condition. It is difficult to estimate the risk for any one person or family, since there are various factors that make family members alike and different. If you share genetics but eat different diets and live different lifestyles, your risks will be different.
If you have a family history of Alzheimer’s disease or questions about genetic testing for the condition, a genetic counselor may be able to help. Learn more about genetic counseling.
Find Out if Your Genetics Might Increase Your Likelihood for Developing Alzheimer’s.
Early-Onset Alzheimer’s genes
Early-onset Alzheimer’s disease is strongly influenced by genetics. Three genes, Amyloid precursor protein (APP), Presenilin 1 (PSEN1), and Presenilin 2 (PSEN2), are known to be factors in how the body produces and uses a protein fragment called beta-amyloid. It is not known exactly how genetic variants increase the risk of early-onset Alzheimer’s disease. However, beta-amyloid is known to impact the lifespan and function of brain cells.
Late-Onset Alzheimer’s genes
Late-onset Alzheimer’s disease is influenced by genetics of the A P O E gene, but genetics is only one factor in the risk. The A P O E gene contains instructions for making a protein called apolipoprotein E. This protein helps control the levels of cholesterol and fats in the blood. It is not known exactly how the ε4 variant increases the risk of late-onset Alzheimer’s.
Having the ε4 variant in A P O E does not mean that you have or will develop late-onset Alzheimer’s disease in your lifetime. How much your risk increases varies on a number of different factors such as your sex, age, and number of e4 variants. Researchers believe that a combination of genetic, environmental, and lifestyle factors all play a role.
A P O E Prevalence by Sex
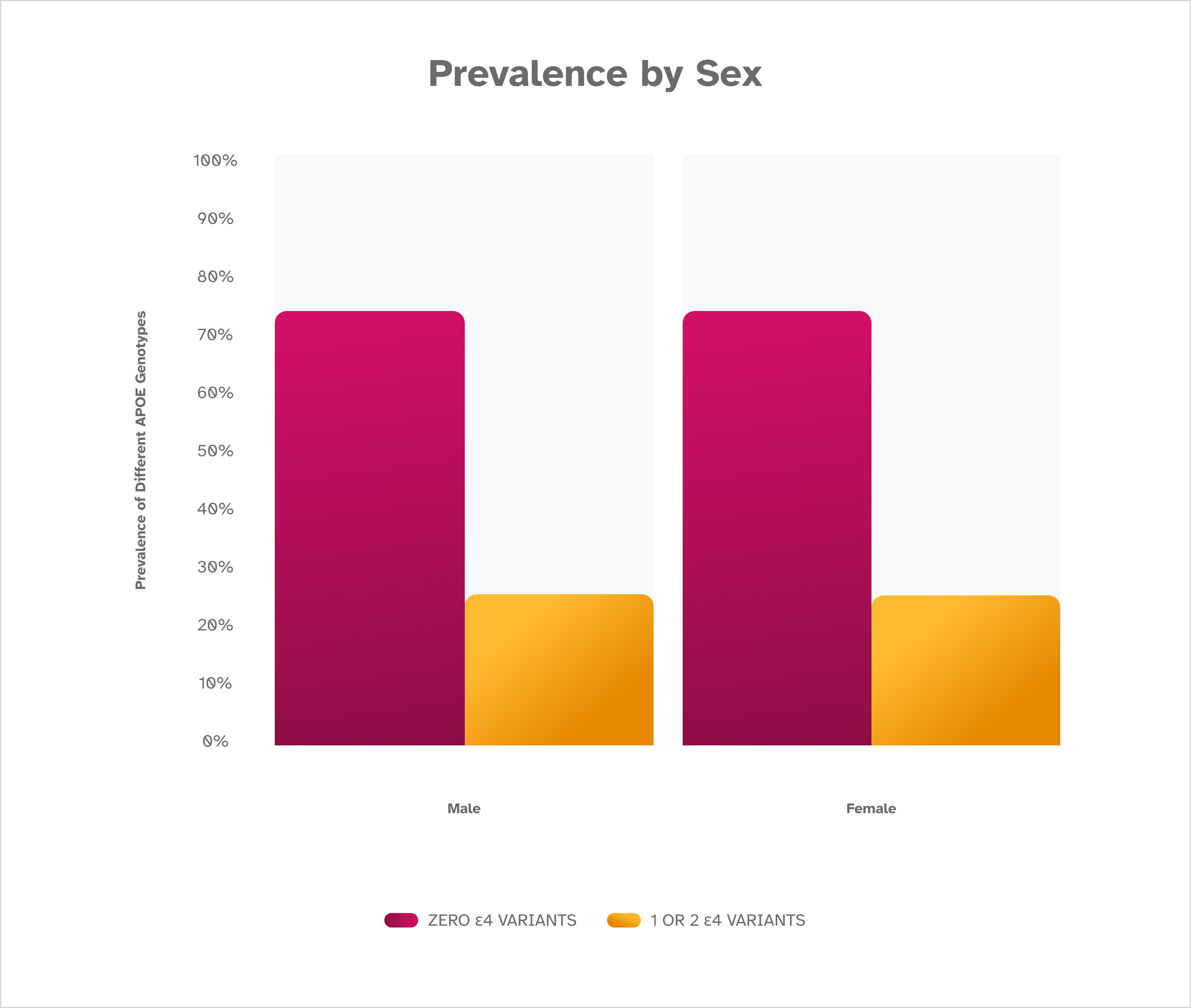
As expected, the prevalence of the ε4 variant is the same regardless of sex. This suggests other factors beyond the ε4 variant are at play in the different rates of Alzheimer’s disease noted between males and females. This is based on December 2022 data from consented 23andMe research participants of European descent.
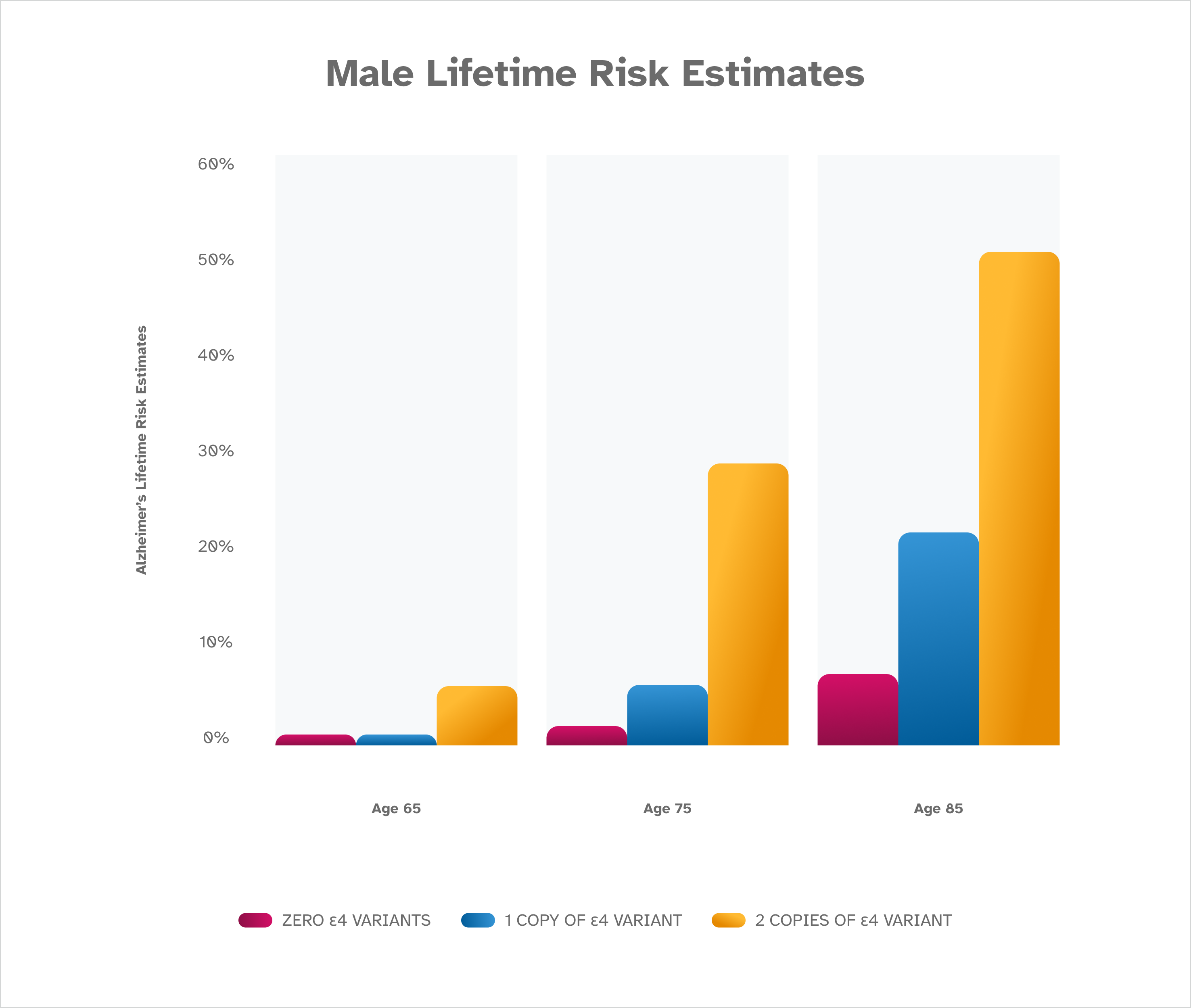
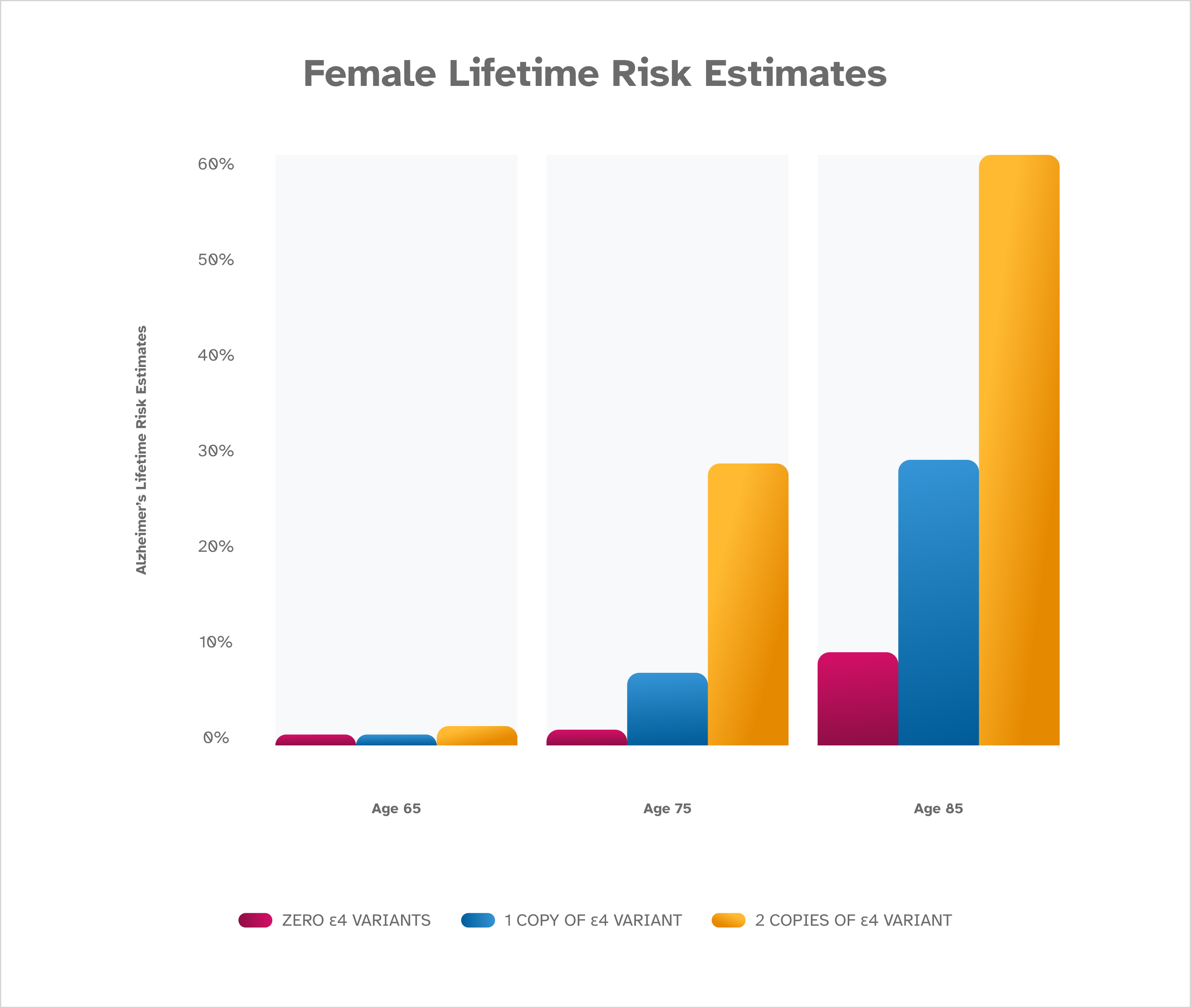
The risk of Alzheimer’s disease increases with age, and is highest for females. The presence of an ε4 variant also increases risk, with two copies of ε4 leading to the highest risk for both males and females. These risk estimates are from Genin et al. [2011] and are based on people of European descent. Lifetime risk estimates are not available for people of other ethnicities.
What other risk factors for Alzheimer’s disease are there?
Non-genetic factors are associated with higher or lower risk for Alzheimer’s. Many of these factors are interrelated — diet, exercise, and heart health, for instance. Here’s what we know about each one:
Age
The risk of Alzheimer’s disease increases greatly as a person ages. This condition is most often diagnosed in people over the age of 65.
Sex
More females than males have late-onset Alzheimer’s disease. This may be partly due to the fact that women tend to live longer than men, but biological and lifestyle differences likely also play a role.
Family History
Family history also matters for Alzheimer’s disease. People who have a parent or sibling with Alzheimer’s are more likely to develop the condition. This could be due to shared genetics, but it could also reflect a shared home environment and lifestyle, or a combination.
Heart Health
Many studies have investigated the relationship between cardiovascular risk factors and Alzheimer’s disease. Evidence suggests that factors that increase the risk of cardiovascular disease (obesity, high cholesterol, high blood pressure, type 2 diabetes, and smoking) also increase the risk of Alzheimer’s disease.
Diet
Understanding the effects of diet on Alzheimer’s disease risk is an active area of research. Studies suggest a diet with plenty of green leafy vegetables, fruits, whole grains, and healthy fats such as those found in fish, nuts, and olive oil is associated with a reduced risk of developing Alzheimer’s disease.
Exercise
Understanding the effects of exercise on Alzheimer’s risk is an active area of research. Evidence suggests that exercise benefits the brain and decreases the risk of developing Alzheimer’s disease.
Intellectual activity
Fewer years of education has been associated with a greater risk of developing Alzheimer’s disease later in life. The cause of this association is unclear. It could be that lower education levels reflect lower socioeconomic status, which may limit a person’s access to affordable health care and nutritious food.
Race and Ethnicity
Late-onset Alzheimer’s disease affects people of all races and ethnicities. About 1 in 10 Americans age 65 and older is affected by Alzheimer’s disease. Elderly African Americans and Hispanic/Latin Americans are more likely to develop the condition than people of other races and ethnicities. For reasons not yet fully understood, other genetic factors in addition to the A P O E gene and e4 variant – and non-genetic factors – seem to contribute to a higher risk of late-onset Alzheimer’s disease among these population groups.
Can genetic testing tell me if I am at risk for Alzheimer’s disease?
If you have a family history of Alzheimer’s disease or think you have symptoms, consult with a healthcare professional. Some healthcare professionals such as genetic counselors can answer questions about genetic testing for Alzheimer’s disease and discuss your options for testing in a clinical setting using either blood or saliva.
Genetic testing for late-onset Alzheimer’s disease differs from the genetic testing for early-onset Alzheimer’s disease. One uses a technology called genotyping and the other uses genomic sequencing.
The 23andMe PGS test uses genotyping technology. You can find out whether you may have an increased risk of developing late-onset Alzheimer’s disease based on your genetics with the 23andMe Late-Onset Alzheimer’s Disease Genetic Health Risk report*. The report looks for the ε4 variant in the A P O E gene associated with late-onset Alzheimer’s disease. The report is available through the 23andMe Health + Ancestry Service. Having a higher risk for late-onset Alzheimer’s disease does not mean that you have or will develop the condition.

Health + Ancestry Service
*The 23andMe PGS test uses qualitative genotyping to detect select clinically relevant variants in the genomic DNA of adults from saliva for the purpose of reporting and interpreting genetic health risks. It is not intended to diagnose any disease. Your ethnicity may affect the relevance of each report and how your genetic health risk results are interpreted. Each genetic health risk report describes if a person has variants associated with a higher risk of developing a disease, but does not describe a person’s overall risk of developing the disease. The test is not intended to tell you anything about your current state of health, or to be used to make medical decisions, including whether or not you should take a medication, how much of a medication you should take, or determine any treatment. The Late-Onset Alzheimer’s Disease genetic health risk report is indicated for reporting of the ε4 variant in the A P O E gene and describes if a person has a variant associated with an increased risk of developing late-onset Alzheimer’s disease. The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Can genetic testing diagnose Alzheimer’s disease?
If you are concerned about symptoms, it’s important to talk to your doctor or healthcare professional. A genetic test cannot diagnose you with Alzheimer’s, it provides information on whether your risk is elevated or reduced compared to other people.
A doctor evaluating someone with early signs of Alzheimer’s disease may do cognitive tests and then refer them to a specialist for further testing. Some of those additional tests may include brain imaging and/or lab tests to look for signs of the disease, which can include structural changes in the brain or changes in levels of certain types of proteins.
As part of its Healthy Brain Initiative, the CDC emphasizes the importance of early detection, which gives people and their healthcare providers information, care, and support for their diagnosis.
How can I reduce my risk for developing Alzheimer’s disease?
There is currently no cure for Alzheimer’s disease, and because of this some people decide not to find out about their genetic risk for Alzheimer’s. Other people will decide to be tested because medication may be used to delay or ease symptoms, and there are also lifestyle changes that address Alzheimer’s risk factors.
-
- Diet — Studies suggest a diet with plenty of green leafy vegetables, fruits, whole grains, and healthy fats such as those found in fish, nuts, and olive oil is associated with a reduced risk of developing Alzheimer’s disease.
-
- Intellectual Activity — Researchers suggest that exercising the brain through activities like reading, writing, and doing puzzles may help promote brain health.
-
- Exercise — Evidence suggests that exercise benefits the brain and may decrease the risk of developing Alzheimer’s disease. This may result from many factors, including improvements in blood flow and a lower risk of developing metabolic and cardiovascular diseases.
-
- Heart Health – There is evidence that reducing hypertension — high blood pressure — reduces the chance for cognitive impairment in older adults. So taking preventive steps to control high blood pressure through diet, exercise, and, when appropriate, medication to lower blood pressure, could help lessen the risk for dementia later in life.
Consult with a healthcare professional before making any major lifestyle changes.
Can knowing that I have the e4 genetic variant tell me anything else about my health?
There is currently no way to prevent Alzheimer’s disease. However, understanding more about the condition could help you or someone you know.
If you find out you have one or two copies of the e4 genetic variant in A P O E, talk to your healthcare provider to determine what steps you can take to improve your brain and heart health and reduce your risk of developing Alzheimer’s disease. There is growing evidence that healthy behavior change may help reduce the risk for cognitive decline and possibly dementia. For example, an NIH study showed that lowering systolic blood pressure could help reduce mild cognitive impairment.
Is there any more research being done on the ε4 genetic variant?
Understanding more about the A P O E gene and the ε4 variant can help in more ways than one. Genes are often active in more than one type of body cell, and A P O E is no exception. Research by 23andMe and others is revealing associations between the A P O E ε4 variant and other things that can impact health, like lipid levels in the blood.
Lipids are fats that are important to the body’s function. Having too much or too little of certain lipids can have negative impacts. 23andMe research has found associations between the ε4 variant and undesirable levels of certain lipids. An undesirable balance of lipids can impact both brain and blood vessel health, but it’s an area where interventions and treatments may help.
The following graphs on the ε4 variant and LDL, HDL, and triglycerides are based on 23andMe research data.
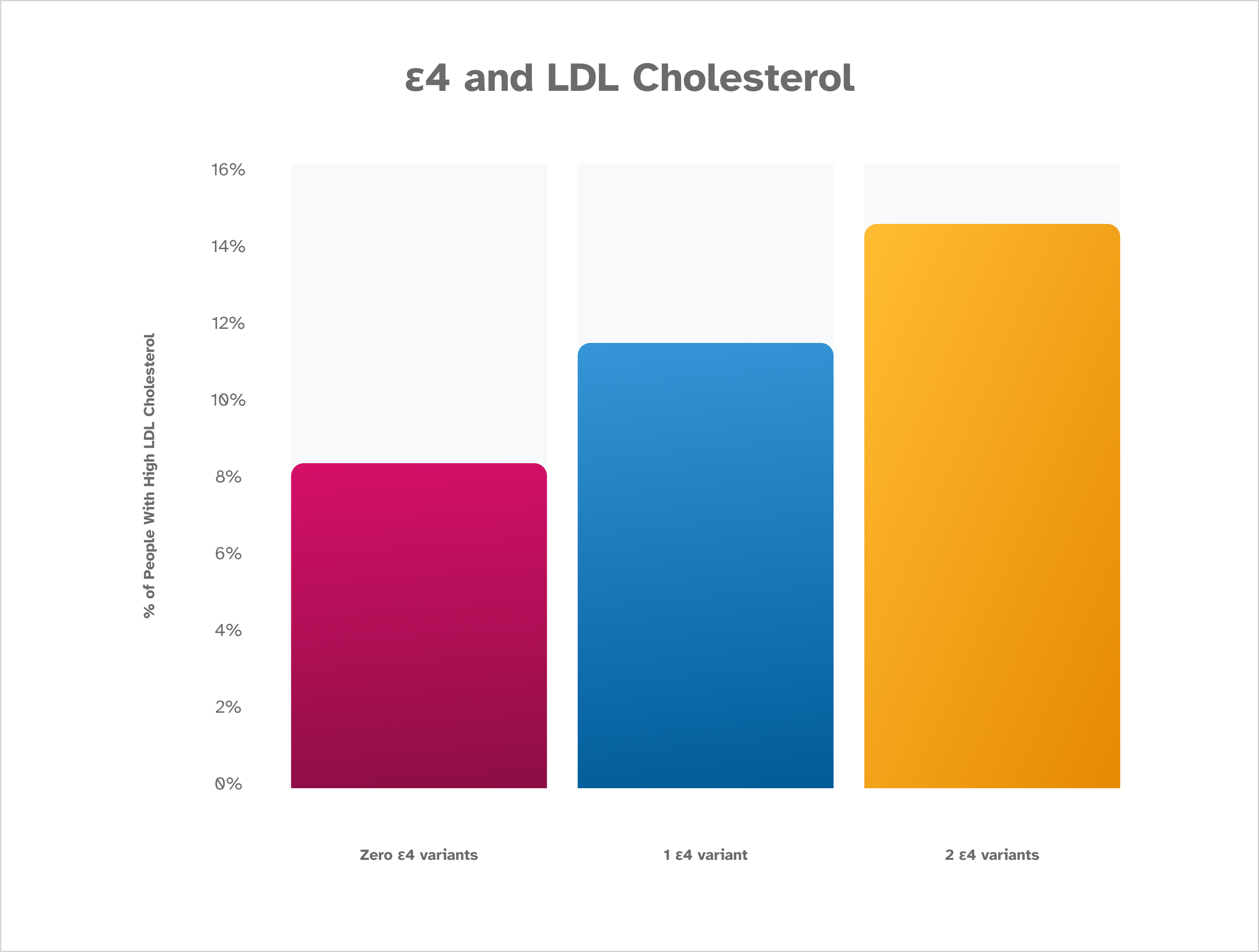
The percentage of people reporting high LDL cholesterol increases along with the number of ε4 variants that are detected. Those who have two ε4 variants are the most likely to report high LDL cholesterol, while those who do not have an ε4 variant identified are least likely to report high LDL cholesterol. This is based on January 2023 data from about 200,000 consented 23andMe research participants.
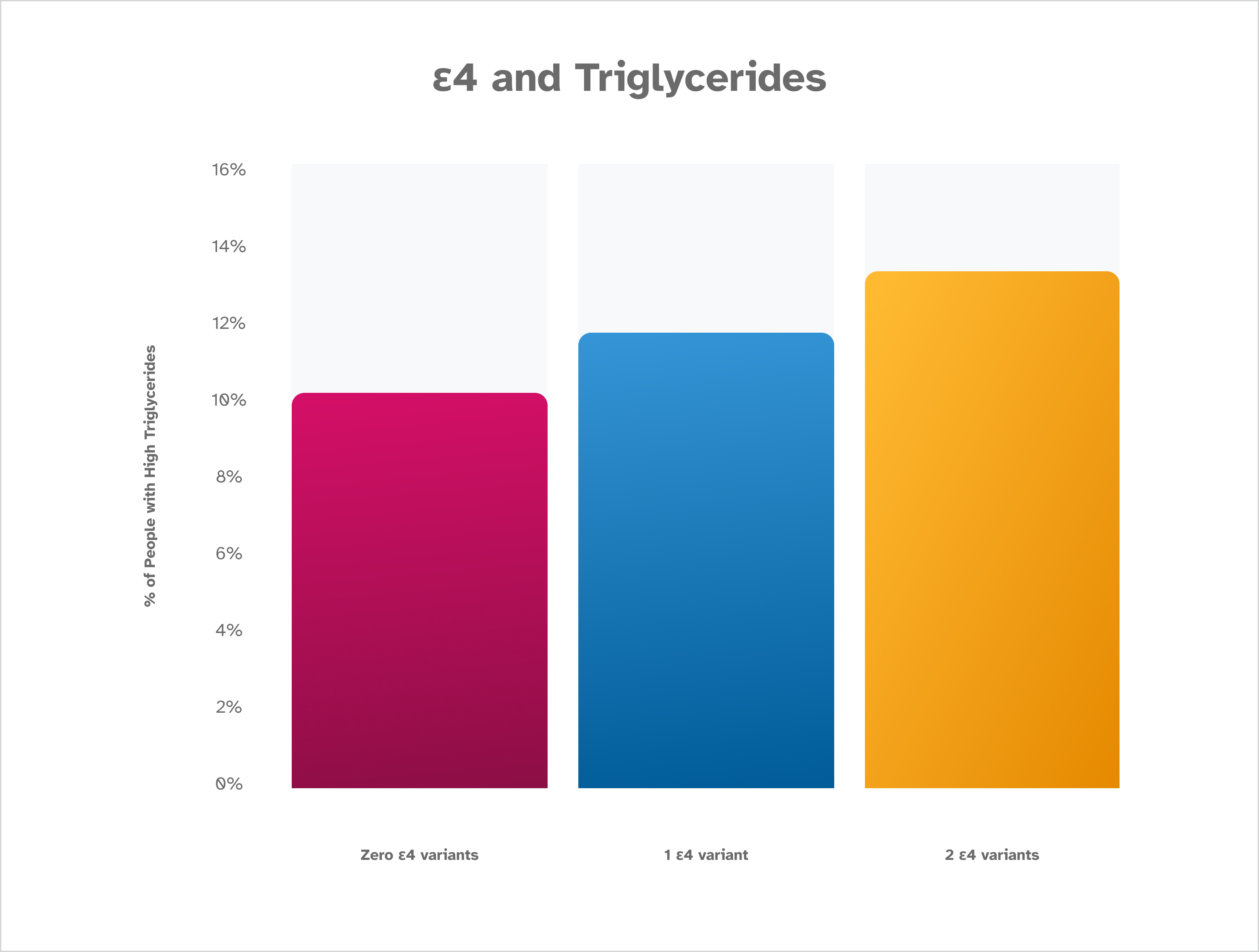
Similar to what is seen with LDL, the percentage of people reporting high triglycerides increases along with the number of ε4 variants that are detected. Those who have two ε4 variants are the most likely to report high triglycerides. Those who have zero ε4 variants identified are the least likely to report elevated triglycerides. This is based on January 2023 data from about 200,000 consented 23andMe research participants.
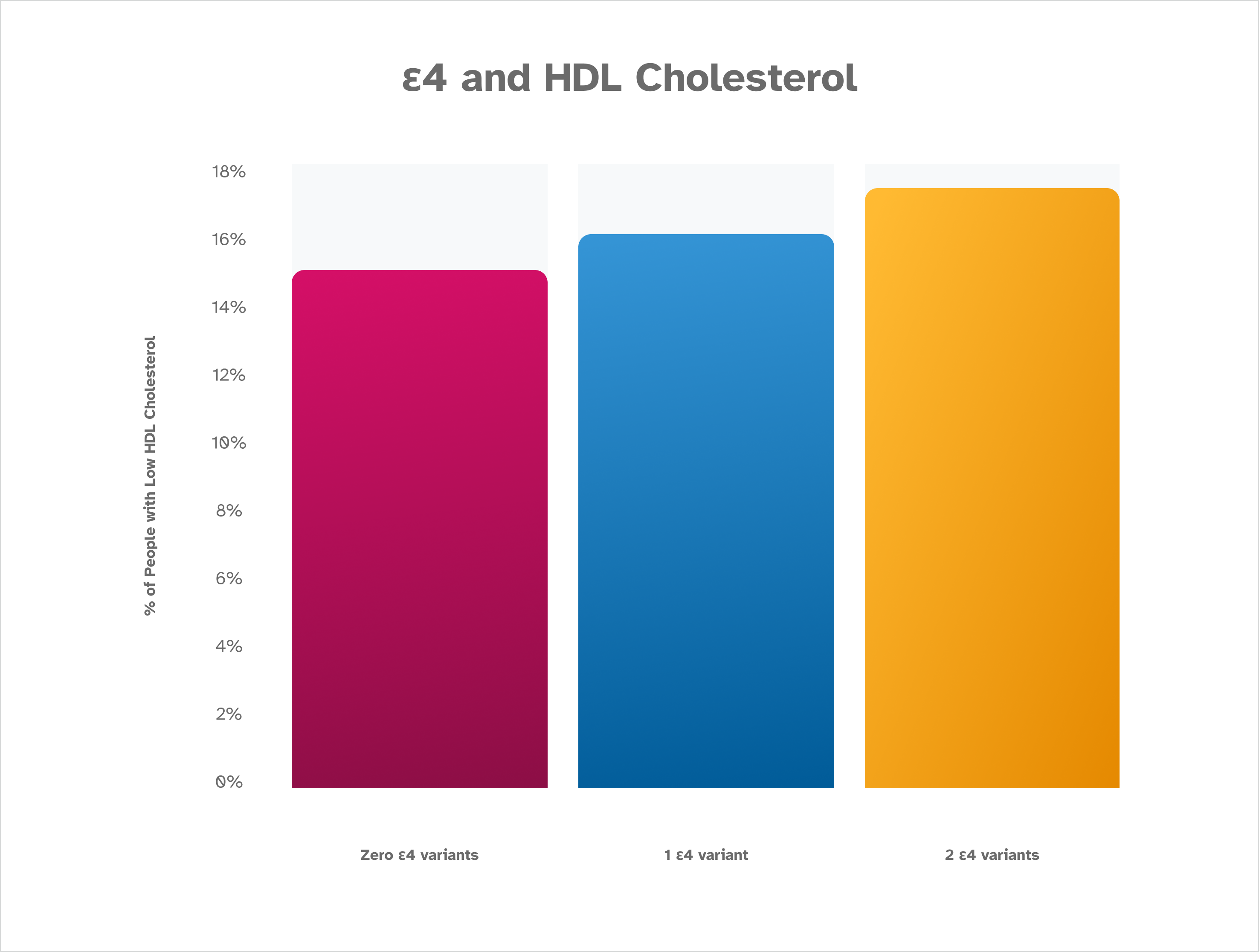
The A P O E ε4 variant is associated with low HDL cholesterol levels, which is the inverse pattern of that seen with LDL and triglyceride levels. This makes sense, because HDL is often thought of as a “healthy” cholesterol. Higher levels of HDL cholesterol are desirable. This is based on January 2023 data from about 200,000 consented 23andMe research participants.
How else can knowing ε4 status help? Researchers are still working to understand more. Information collected from consented research participants at 23andMe, like whether they smoke or play a musical instrument, are helping build knowledge around the factors at play in cognition overall.
23andMe measured cognitive ability of research participants using the Digit Symbol Substitution Test (DSST) which is a highly accepted metric in the research community. For all participants — including those with and without the ε4 variant — an association was found between cognitive ability and certain lifestyle factors, like smoking and playing a musical instrument.
As expected, cognitive ability (DSST) declined with age and participants that have Alzheimer’s disease have lower DSST scores (data not shown). Additionally, people who have higher cognitive ability are less likely to have a history of smoking and are more likely to play a musical instrument, compared to people with lower cognitive ability.
What 23andMe researchers and other researchers do not yet fully understand is the reason for these associations and the role of confounding factors. It will take more data on diverse groups of people – as well as ruling out other causes – to fully understand cause and effect.
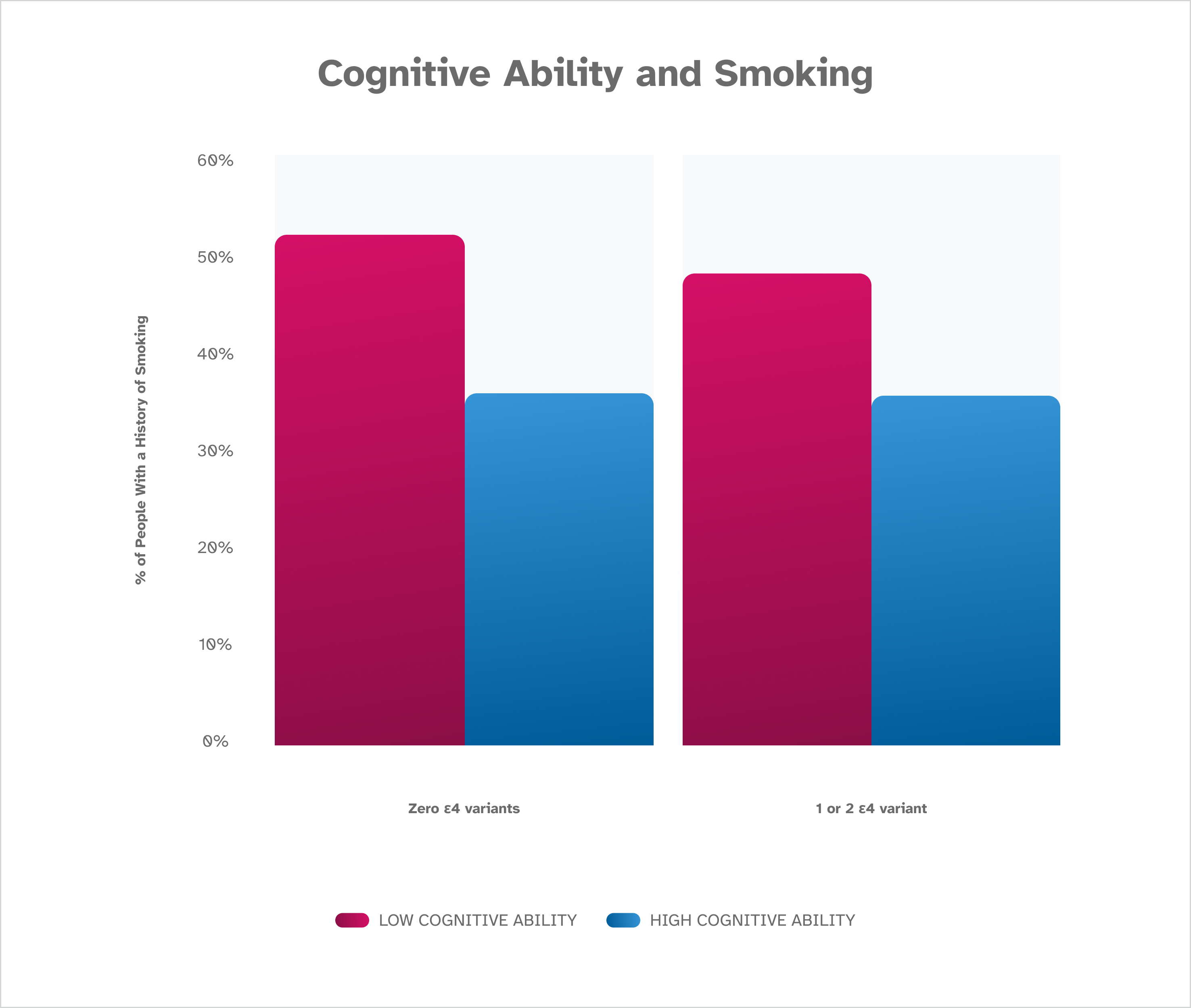
These data show an association between smoking and cognitive ability and reveal that research participants who report having never smoked have higher scores on the Digit Symbol Substitution Test (DSST) than research participants who report a history of smoking. The finding holds true whether a participant has zero, one, or two A P O E ε4 variants. These findings are based on January 2023 data from about 80,000 consented 23andMe research participants over the age of 60.
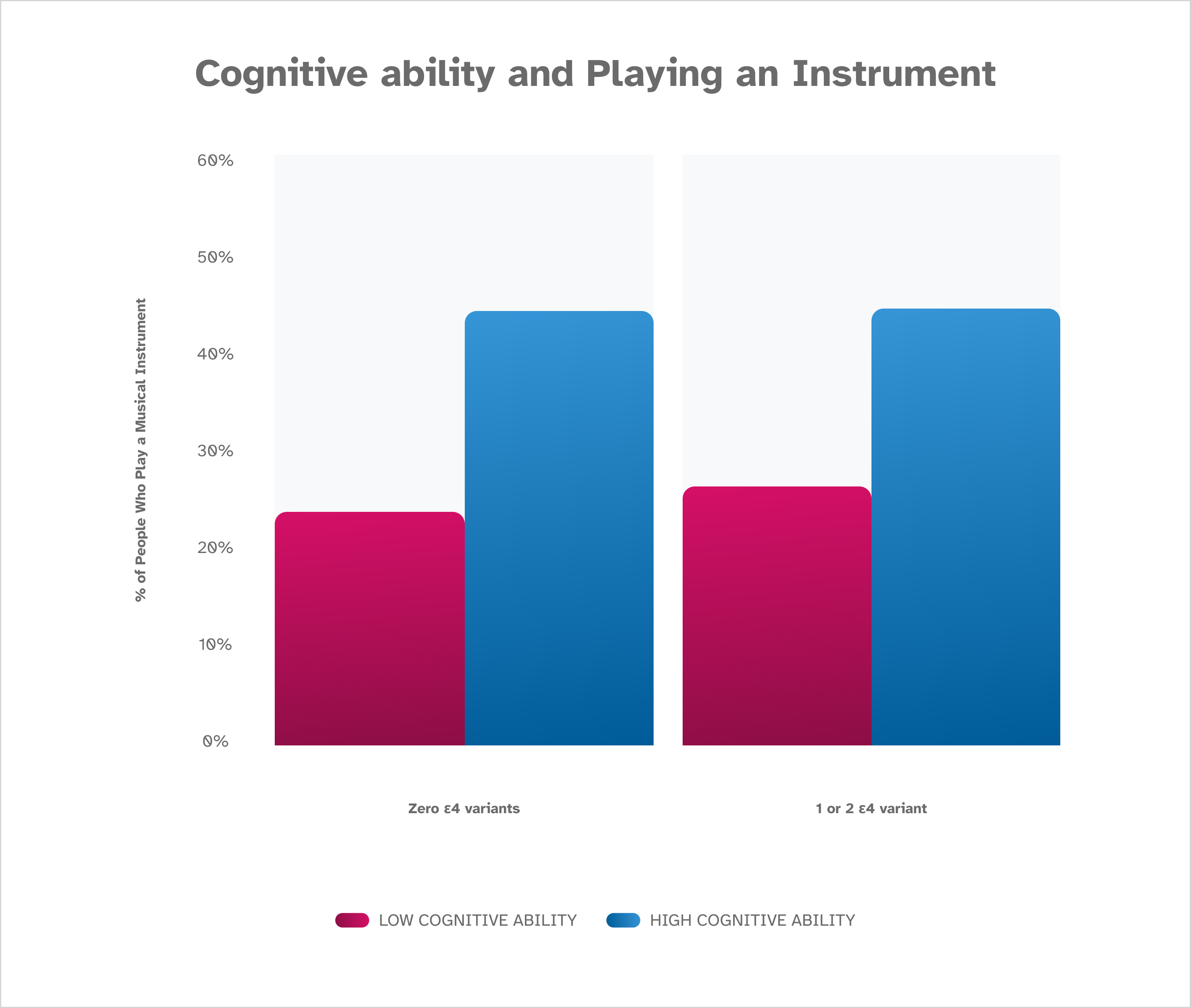
These data show an association between playing a musical instrument and cognitive ability and reveal that research participants who report playing a musical instrument have higher scores on the Digit Symbol Substitution Test (DSST) than research participants who report they do not play a musical instrument. The finding holds true whether a participant has zero, one, or two A P O E ε4 variants. These findings are based on January 2023 data from about 80,000 consented 23andMe research participants over the age of 60.
References
Bird TD et al. (1998) “Alzheimer’s Disease Overview. [Accessed Nov 9, 2021].